Introduction
There are no recommendations pertaining to lymphoma in AYA population. It represents a paramount opportunity for fostering collaborative research among pediatric and medical oncologists. Prior to embarking on any collaborative interventional research, comprehensive demographic data, patterns of care, and outcomes related to AYA lymphomas are imperative. Hence, the HCC registry database can be utilized to do this analysis of all AYA lymphoma in India which will set the benchmark for future research in this area.
Methods
This was a retrospective study including data from participating member centers of the HCC registry. Data was collected using an online data capture program, which is already in place as part of the HCC registry and has been in use for the past 5 years. Each center maintains its source documents and is responsible for data accuracy. All patients diagnosed with lymphoma between January 01, 2020, and June 31, 2022, were included in the study. The treatment patterns for AYA lymphomas vary from intensive protocols like in the pediatric age group to less intense protocols used in adults. This registry data captured this heterogeneity in treatment patterns and outcomes. The primary endpoint of the study was event-free survival (EFS) which was defined as the time from the first cycle of chemotherapy to progression, relapse, second malignancy, or death. The secondary outcomes were response rates and overall survival.
Results
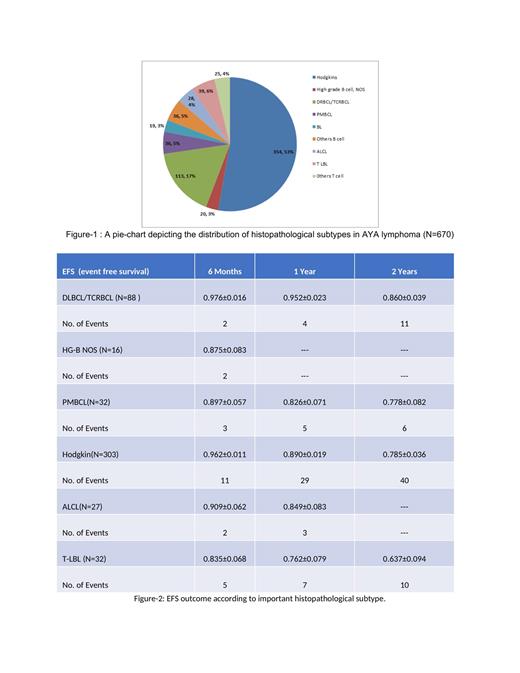
The data was collected from eight participating centers of India out of which five are public sector hospitals and three are trust-based private hospitals. A total of 677 AYA lymphomas were registered between January 01, 2020, and June 31, 2022. Treatment was given to 582 patients and the follow-up data was available for 570 patients. The median age of the cohort was 23 years (IQR-19, 27) with 65% of them being males. Almost 50 % of patients had Hodgkin lymphoma and the rest were non-Hodgkin lymphoma (NHL) as depicted in Figure 1. Among NHLs; DLBCL, T-LBL, PMBCL, ALCL, and Burkitt lymphoma were seen in 113(17%), 39(6%), 36(5%),28(4%), and 19(3%) respectively. PETCT was done in 70% patients at baseline. Early-stage disease was seen in 233 patients (35%), advanced stage in 366 patients (56%), and 60 patients (9%) were not staged at baseline. Bulky mediastinum was seen in 1/4 th of the patients out of which the majority (85%) were Hodgkin lymphoma and PMBCL. Extra nodal site involvement was seen in 43% of patients out of which marrow involvement constituted almost half. Out of 677 patients, 582 patients (86%) received treatment at their respective centers and only 5 % of patients could not receive any treatment due to financial constraints. Among 298 treated cases of Hodgkin lymphoma, 245(82 %) patients received ABVD while escBEACOPP was given to 28(9%) patients. In DLBCL, out of 87 patients, 59(68%) received CHOP-R, 10(11%) received DA-REPOCH, 8(9%) received GMALL NHL protocol and the rest 10(11%) received other intensive regimens. Around one-third(36%) patients received radiation therapy out of which 90 patients (57%) received it as part of definitive treatment and 50 (31%) patients received it as consolidation to residual site. The median follow-up of the entire cohort was 13 months(IQR-8, 12). Out of 570 patients where follow-up was available, the overall EFS was 76. % ± 2.4%. The EFS outcome according to the histopathological subtype is depicted in Figure 2. Relapse or progression occurred in 72 patients (12.5%) out of which 50(72%) could receive any form of salvage therapy including transplant. The most common cause of death was progressive disease in 18 patients (50%) and death due to toxicity in 3(8%), and unknown causes in 16(40%) patients. The presence of bulky disease or bulky mediastinum did not have an impact on EFS outcome.There was no difference in EFS between DLBCL or High-grade B- NHL NOS.
Conclusion
This is the one of largest real-world data on AYA lymphoma. The majority were Hodgkin lymphoma followed by DLBCL. Most patients received adult-type regimens or Hodgkin as well as non-Hodgkin lymphoma. The use of radiation was high (1/3 rd) which could be due to a higher burden of bulky and mediastinal disease in our cohort. The EFS outcomes are comparable to the published literature. This data will set the benchmark for future collaborative studies in India and abroad for AYA lymphoma.
Disclosures
Jain:Intas Pharmaceuticals: Research Funding; Zydus Pharmaceuticals: Research Funding; ImmunoACT: Research Funding. Abraham:Pfizer: Research Funding; Sanofi: Research Funding; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Research Funding.